Core research themes
These are the endothelial cells derived from small vessels or capillaries: the finest branches of the vascular tree. Here, the vascular wall consist of nothing but these endothelial cells and a basal lamina together with a few scattered mural cells called pericytes.
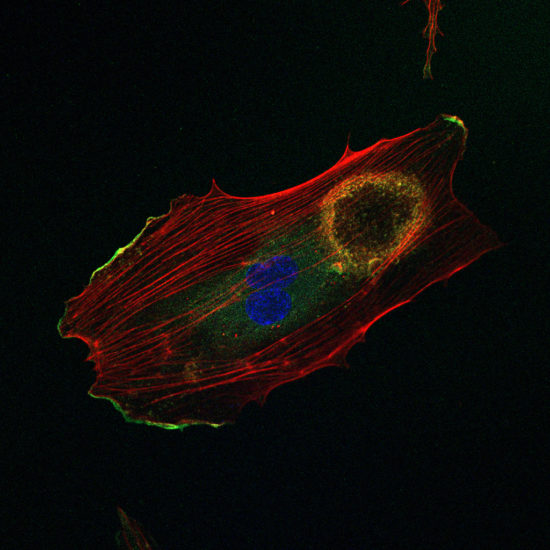
Fig. 8. In cultured microvascular endothelial cells, VEGF induces the assembly of podosomes that arrange in a ring-shape superstructure.
Microvascular endothelial cells also assemble podosomes, but this is a reponse to angiogenic stimulation, i.e., upon exposure to VEGF. Like for TGFβ-treated arterial endothelial cells, VEGF induced-podosomes also arrange in rosettess (Fig. 8). The presence of the extracellular matrix proteins laminin and collagen-IV is essential for the VEGF response. Moreover, only in presence of collagen-IV are these podosomes proteolytically active. The activation pathway involves phosphorylation of Src, relocalisation of 190RhoGAP-B (also known as ARHGAP5) and exposure of MT1-MMP (also known as MMP14) at the cell surface. We conclude from this that the angiogenic potential of VEGF resides in part in podosome-mediated degradation of the basement membrane, by enabling endothelial cells to pass anatomical barriers.
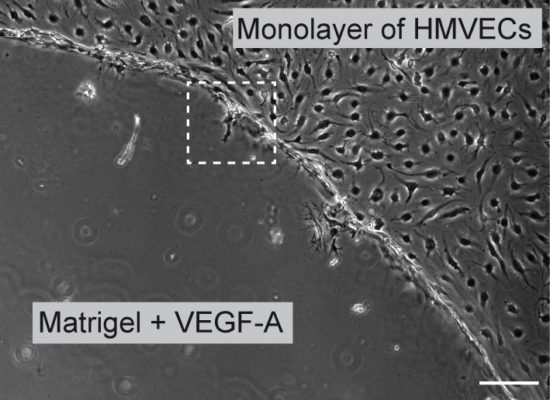
Fig. 9. An in vitro invasion angiogenesis assay. We have set up a novel assay with which we can induce tip cell/stalk cell specification in vitro and study their behaviour by videomicroscopy
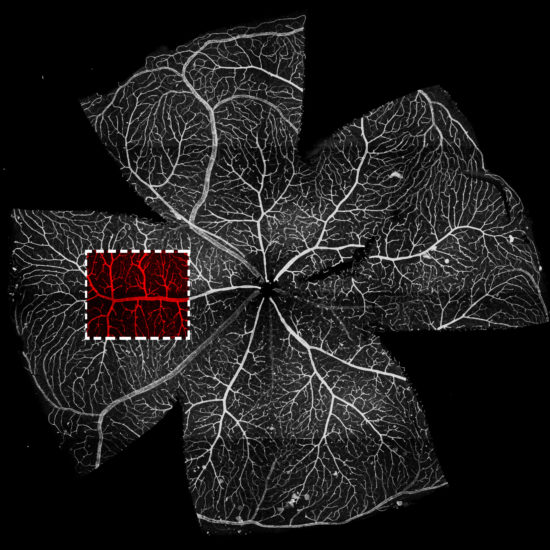
Fig. 10. An in vivo angiogenesis assay. The post natal retina is used to study angiogenesis in vivo.
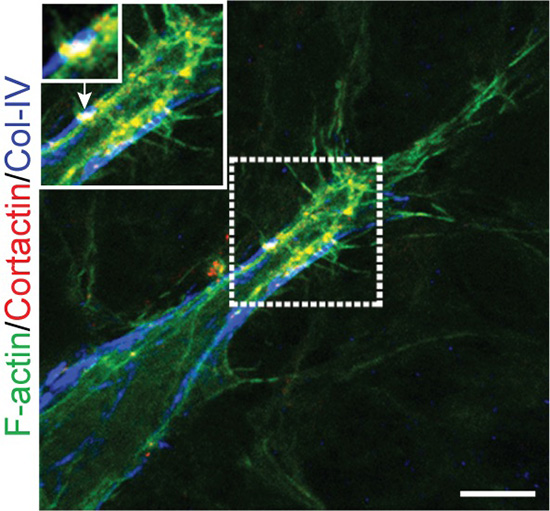
Fig. 11. A tip cells with podosomes, in vivo, visualized at the angiogenic front in the mouse retina model. The image shows the merge of 3 stainings: the actin cytoskeleton is shown in green, collagen-IV in the basement membrane iis stained in blue, the podosomal marker cortactin appears in red (scale bar is 10 micrometers).
VEGF is the main regulator of angiogenesis, the process by which the blood vessel network expand from the existing microvasculature. Expansion occurs in a two-step process that involves sprouting and anastomosis, and culminates in the formation of a functionally perfused vascular bed. In the sprouting phase, outgrowing capillaries are guided by specialized endothelial cells termed tip cells toward VEGF gradients produced by tissues that are low in oxygen supply (hypoxic). Tip cells explore their environment by extending dynamic filopodia and migrate in response to VEGF signals. Concomitantly neighboring endothelial cells are prevented from doing so and instead are instructed to become stalk cells forming the trunk of the neo-vessel. Stalk cells are exposed to relatively lower levels of VEGF to which they respond by proliferation. The loops formed by these growing sprouts connect to the rest of the network by anastomosis. Tip cell/stalk cell specification is controlled by the Notch pathway initiated by VEGF. Notch activity is low in tip cells and high in stalk cells.
Using a novel in vitro angiogenesis assay mimicking the tridimensional microenvironment that endothelial cells encounter in vivo, we showed that VEGF/Notch signaling regulates the formation of functional podosomes in endothelial cells. These studies also established that the microenvironment regulates podosome arrangement in angiogenic endothelial cells. In this setting, podosomes appear as globular collagenolytic structures, interconnected by actin filaments, along cellular protrusions that endothelial cells form when embedded in a 3D matrix (Fig. 9).
The interplay between tip cells and stalk cells is well described in the developing retinal vasculature of the neonatal mouse (Fig. 10). We used this neovascularization model to detect podosomes and found them in tip cells at the forefront of the endothelial sprout in vivo (Fig. 11). In the distal retinal vasculature, podosomes are also part of an interconnected network that surrounds large microvessels and impinges on the underlying basement membrane. Consistently, collagen-IV is scarce in podosome areas. Moreover, pharmacological inhibition of Notch signaling exacerbates both podosome formation and collagen-IV loss. Thus podosome induction relies on tip/stack cell specification and the notch pathway appears as a novel regulatory mechanism of podosome formation. The localized proteolytic action of podosomes, leading to removal of collagen-IV from the basement membrane, appears to facilitate both endothelial cell sprouting and anastomosis within the developing vasculature.
Angiogenesis is a vital process during development and tissue regeneration but a major threat in certain diseases conditions. The identification of podosomes as key components of the sprouting machineries provide another opportunity to target angiogenesis therapeutically. However, the molecular mechanism by which tip cells acquire their morphological and functional features remains unclear. Ongoing studies aim at addressing the role of podosome assembly in the acquisition of the tip cell phenotype: how does reduced Notch activity contribute to actin cytoskeleton remodeling and podosome formation, which signalling pathways are involved in this process, and how actin cytoskeleton remodeling is involved in metabolic adaptation during angiogenic sprouting.