Core research themes
These are the endothelial cells derived from large vessels. The endothelium from which they originate represents an exceedingly thin sheet in comparison to the thick and tough outer layers made of connective tissue and smooth muscle cells. Macrovascular cells are firmly attached onto the underlying basement membrane, a mechanical and anatomical barrier which also promotes endothelial cell homeostasis.
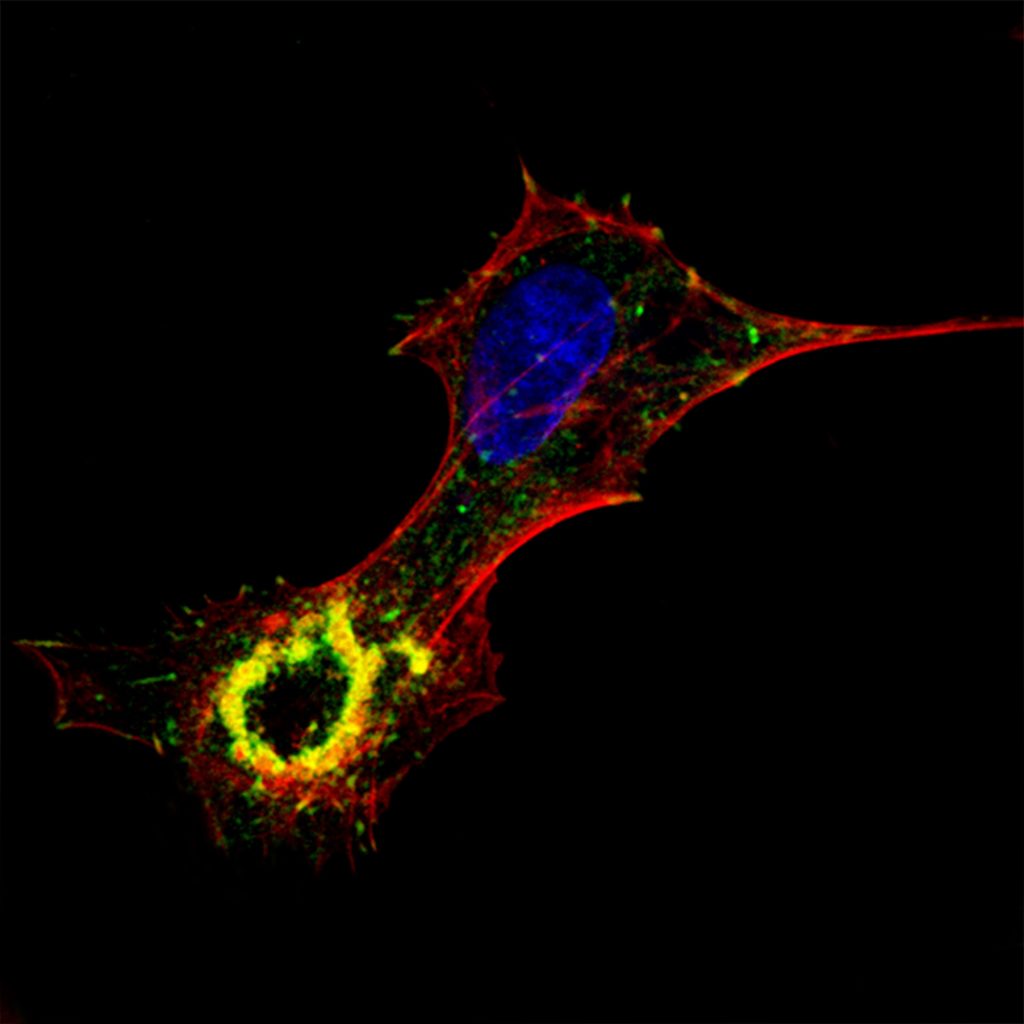
Fig.4. In cultured aortic endothelial cells, TGFß induces the assembly of podosomes that arrange in a ringshape super-structure.
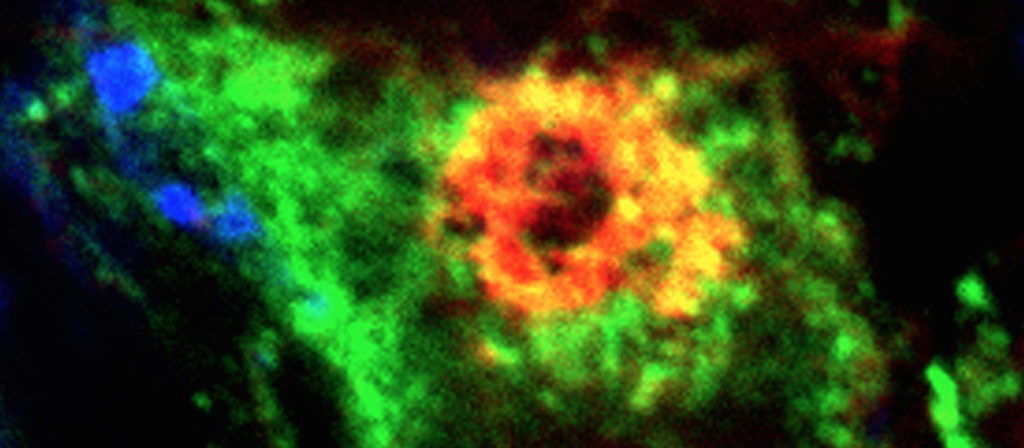
Fig. 5. In the aortic endothelium, TGFß induces the assembly of podosomes that arrange in a ringshape super-structure.
We have revealed that arterial endothelial cells assemble podosomes upon exposure to the growth and differentiation factor TGFß. The podosomes arrange in a ring-shape super-structure that is often called podosome rosette. Rosettes have been observed in cultured cells (“in vitro”) (Fig. 5) as well as in the aortic endothelium (in situ, ex vivo)(Fig. 6). In both situations, we have demonstrated that collagen-IV, a key component of the basement membrane, is degraded underneath podosome rosettes. As TGFß has an important role in the development and maintenance of the vascular system, podosomes may play a role in the remodeling of vessels under physiological as well as pathological conditions.
Ongoing projects aim at demonstrating the existence of podosomes in the aortic endothelium in vivo and to establish their role in physiological and pathological arterial remodeling. We are studying the consequences of endogenous TGFß overproduction/activation on aortic endothelial cells and vessel wall integrity in a mouse model. This has implications in human diseases associated with increased levels of TGFß in the aortic wall in the context of TGFß associated vascular disorders. We hope to learn how to harness endothelial cell behavior so that aberrant vascular remodeling is avoided, and patients with vascular disease have better outcomes.
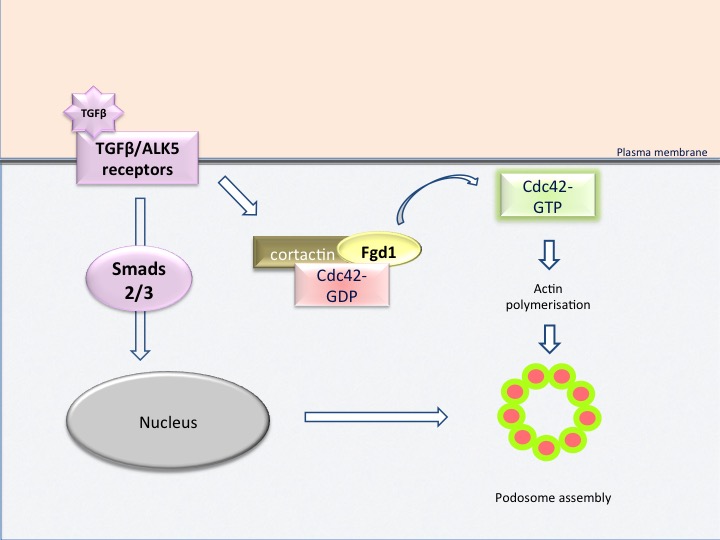
Fig. 6: cartoon illustrating how signaling pathways cooperate to drive podosome rosette assembly in aortic endothelial cells
Our studies have revealed an essential role of GTPase Cdc42 in the induction of podosomes. In this particular situation, the activation of Cdc42 by TGFβ is placed under the control of the exchange factor Fgd1. Activation of Fgd1, in turn, requires phosphorylation on a tyrosine residues by the protein kinase Src. This leads to translocation of Fgd1 to the submembrane cortex, by a mechanism dependent on cortactin, thus establishing the nucleation site for podosome-assembly. A constitutively active form of Fgd1 is sufficient to induce the formation of podosomes. Fgd1 thus appears as a pivotal molecule of the whole process (Fig. 6) and therefore as a potential therapeutic target. Parallel studies have shown that Fgd1 is also a master regulator of invadopodia formation in cancer cells. We are currently investigating the molecular mechanisms by which Fgd1 leads to podosome/invadopodia formation. Fgd1 has been characterised in osteoblast cells in the skeletal system. Current work focuses on the regulation of Fgd1 in endothelial cells by taking advantage of the knowledge acquired in the skeletal system. This line of research should provides a better understanding of the role Fgd1 in podosome formation in endothelial cells and in the biology of bone cells.
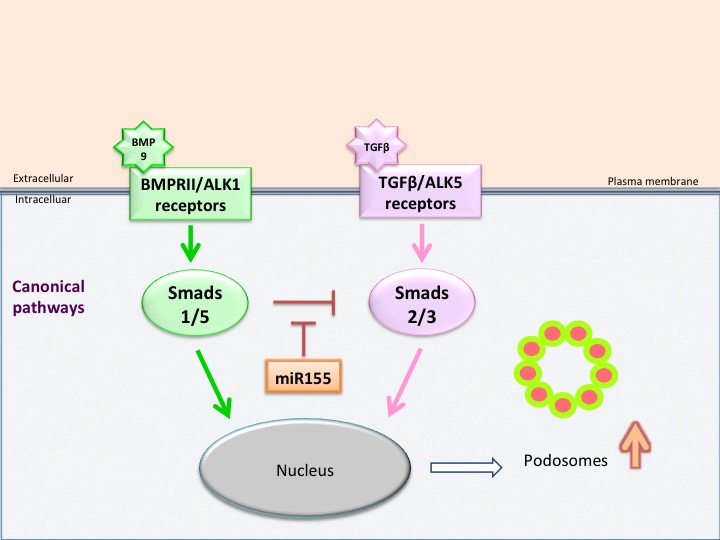
Fig. 7: cartoon illustrating how Smads and miR-155 regulate podosome rosette assembly in aortic endothelial cells
TGF-β drives podosome formation through the TGFβ receptor ALK5 and the downstream effectors Smad2 and Smad3. Concurrent TGFβ-induced ALK1 signaling mitigates ALK5 responses through Smad1. ALK1 signaling induced by BMP9 also antagonizes TGFβ-induced podosome formation, but this occurs through both Smad1 and Smad5. A physiological situation in which BMP9 signals may be impaired occurs in pathological situations associated with miR-155 overexpression, a condition that may occur in cardiovascular diseases. In cells expressing miR-155, Smad1 and Smad5 expression are reduced, podosome formation is induced and cells become hyperresponsive to TGFβ, and as a consequence show excessive rosette formation and matrix degradation (Fig. 7). Thus, miR-155 appears to be a novel player in the signaling pathways controlling podosome formation in aortic endothelial cells. Such specific features of ALK1 and ALK5 signaling have potential clinical implications that are currently under investigation. We are also exploring the consequences of miR-155 overexpression in the vascular system.